OCS™ Lung
The only FDA approved device for both standard and expanded* criteria donor lungs for transplantation.
The OCS Lung is a portable perfusion, ventilation, and monitoring system that maintains the organ at a near-physiologic state – allowing physicians to assess and improve the condition of lungs between the donor and recipient sites.
*Expanded criteria is defined as donor lung pairs initially deemed unacceptable for procurement and transplantation based on limitations of cold static preservation.
The Benefits of OCS Lung
The only FDA approved device for both standard and expanded* criteria donor lungs for transplantation.
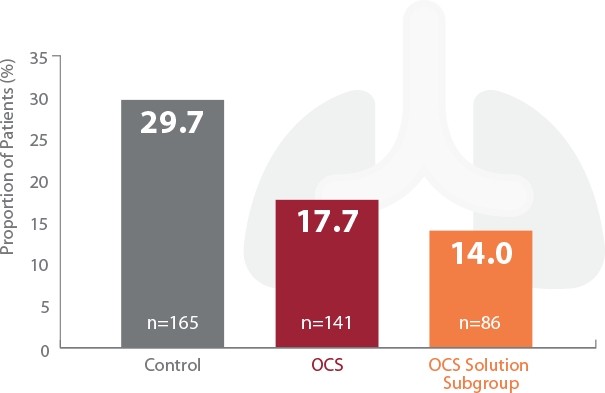
INSPIRE TRIAL RESULTS
Significant Reduction of PGD3 within 72 Hours
†PGD3 in the first 72 hours is a severe form of acute lung injury that is a major cause of early morbidity and mortality encountered after lung transplantation. TransMedics OCS Lung significantly reduced PGD3 vs control group in the INSPIRE Trial.
EXPAND TRIAL RESULTS
*UNOS data showed donor lungs used for the OCS Lung EXPAND Trial had been declined for transplantation on average 35 times by other transplant centers before reaching an OCS transplant center.
Want to learn more about the benefits of OCS, or access our OCS publications repository for additional insights?
Long-distance retrieval
Locate an NOP center near you
University of Alabama at Birmingham
1720 University Blvd
Birmingham, AL 35233
(205) 934-4011
The University of Alabama, University Medical Center
850 Peter Bryce Boulevard
Tuscaloosa, AL 35401
(205) 348-1770
Baptist Health Medical Center-Little Rock
9601 Baptist Health Dr
Little Rock, AR 72205
(501) 202-2000
Banner University Medical Center
1111 E McDowell Rd., 2nd Floor
Phoenix, AZ 85006
(602) 839-9300
Mayo Clinic
5777 E Mayo Blvd
Phoenix, AZ 85054
(480) 342-2000
St. Joseph's Hospital and Medical Center
350 W Thomas Rd
Phoenix, AZ 85013
(602) 406-3000
Mayo Clinic
13400 E Shea Blvd
Scottsdale, AZ 85259
(480) 301-8000
Cedars-Sinai Medical Center
127 S San Vicente Blvd #A6100
Los Angeles, CA 90048
(310) 423-5460
Scripps Green Hospital
9888 Genesee Ave
San Diego, CA 92037
(858) 834-1798
California Pacific Medical Center
3773 Sacramento St
San Fransisco, CA 94110
(415) 600-6000
Loma Linda University Medical Center
11234 Anderson St
Loma Linda, CA 92354
(909) 558-4000
UCSF Medical Center at Mission Bay
1975 4th St
San Francisco, CA 94158
(415) 353-3000
Lucile Packard Children's Hospital Stanford
725 Welch Rd
Palo Alto, CA 94304
(650) 497-8000
California Pacific Medical Center
1101 Van Ness Ave
San Francisco, CA 94109
(415) 600-6000
University of California San Diego Medical Center
9300 Campus Point Drive
La Jolla, CA 92037
(619) 543-6222
University of CA San Francisco Med Center
505 Parnassus Ave
San Francisco, CA 94143
(415) 353-1664
UC Davis Medical Center
4301 X St
Sacramento, CA 95817
(916) 734-2011
Stanford Medical Center
300 Pasteur Drive
Palo Alto, CA 94304
(650) 723-8561
Ronald Reagan UCLA Medical Center
757 Westwood Plaza
Los Angeles, CA 90095
(310) 825-9111
Keck Medicine of USC - Keck Hospital of USC
1500 San Pablo St
Los Angeles, CA 90033
(800) 872-2273
Jacobs Medical Center at UC San Diego Health
9300 Campus Point Dr
San Diego, CA 92037
(858) 657-7000
Lifesharing Donate Life Organization
7436 Mission Valley Rd
San Diego, CA 92108
(619) 543-7225
Lucile Salter Packard Children's Hospital
401 Quarry Rd
Palo Alto, CA 94304
(650) 723-5511
Sharp Memorial Hospital
7901 Frost St.
San Diego, CA 92123
(858) 939-3400
University of California, San Diego
9300 Campus Pt. Dr
San Diego, CA 92103
(858) 657-7000
University of Colorado Health Science Center
13001 E 17th Pl
Aurora, CO 80045
(303) 724-5000
UCHealth University of Colorado Hospital
12605 E 16th Ave
Aurora, CO 80045
(720) 848-0000
Hartford Hospital
80 Seymour St
Hartford, CT 06106
(860) 545-5000
Yale New Haven Transplant Center
800 Howard Ave
New Haven, CT 06510
(203) 785-2565
Hartford HealthCare Heart & Vascular Institute
100 Retreat Ave STE 811
Hartford, CT 06106
(860) 522-5712
MedStar Washington Hospital Center
110 Irving St NW
Washington, DC 20010
(202) 877-7000
Nemours Children's Hospital
1600 Rockland R
Wilmington, DE 19803
(302) 651-4200
Weston Hospital | Cleveland Clinic Florida
2950 Cleveland Clinic Blvd
Weston, FL 33331
(954) 659-5000
AdventHealth Medical Group Transplant Institute
2415 N Orange Ave Suite 700
Orlando, FL 32803
(407) 303-2474
Joe DiMaggio Children's Hospital Foundation
3329 Johnson St
Hollywood, FL 33021
(954) 265-3454
Memorial Regional Hospital
3501 Johnson St
Hollywood, FL 33021
(954) 987-2000
Mayo Clinic
4500 San Pablo Road
Jacksonville, FL 32224
(904) 956-3309
HCA Florida Largo Hospital
201 14th St SW
Largo, FL 33770
(727) 588-5200
UF Health Shands Hospital
1600 SW Archer Rd
Gainesville, FL 32608
(352) 265-0111
Emory University Hospital
1365 Clifton Road Northeast, Building B
Atlanta, GA 30322
(855) 366-7989
Loyola University Medical Center
2160 S 1st Ave
Maywood, IL 60153
(888) 584-7888
Northwestern Medicine Organ Transplant Center
676 N St Clair St 19th Floor, Suite 1900
Chicago, IL 60611
(312) 695-8900
Rush University Medical Center
1653 W Congress Pkwy
Chicago, IL 60612
(312) 947-0100
The University of Chicago Department of Medicine
5841 S Maryland Ave
Chicago, IL 60637
(773) 702-1234
UK HealthCare - University of Kentucky
800 Rose St. First Floor, Suite G100
Lexington, KY 40536
(800) 456-5287
Beth Israel Deaconess Medical Center
110 Francis St
Boston, MA 02215
(617) 632-1070
Boston Children's Hospital
300 Longwood Ave
Boston, MA 02215
(617) 355-6000
Lahey Hospital & Medical Center
41 Burlington Mall Road
Burlington, MA 01805
(781) 744-5100
Massachusetts General Hospital
Main Campus
55 Fruit Street
Boston, MA 02114
(617) 726-2000
Tufts Medical Center
800 Washington St.
Boston, MA 02111
(617) 636-2273
Brigham and Women's Hospital
75 Francis St
Boston, MA 02115
(617) 732-5500
UMass Memorial Medical Center
55 N Lake Ave
Worcester, MA 01655
(508) 334-3452
University of Maryland Medical Center
22 S Greene St
Baltimore, MD 21201
(410) 328-8667
Corewell Health Butterworth Hospital
100 Michigan St NE
Grand Rapids, MI 49503
(616) 391-1774
Henry Ford Jackson Hospital
205 N East Ave
Jackson, MI 49201
(517) 205-4800
Cornell Health William Beaumont University Hospital
3601 W 13 Mile Rd
Royal Oak, MI 48073
(248) 898-5000
Henry Ford Hospital
2799 W Grand Blvd
Detroit, MI 48202
(313) 916-2600
Gift of Life Michigan Recovery Suite
3861 Research Park Dr
Ann Arbor, MI 48108
(866) 500-5801
Spectrum Health Hospitals
330 Barclay Ave NE
Grand Rapids, MI 49503
(866) 989-7999
University of Michigan Hospital
1500 E Medical Center Dr
Ann Arbor, MI 48109
(734) 936-4000
Mayo Clinic Hospital, Methodist Campus
201 W Center St
Rochester, MN 55902
(507) 266-7890
Abbott Northwestern Hospital
800 E 28th St
Minneapolis, MN 55407
(612) 863-3900
Mayo Clinic
200 1st St SW
Rochester, MN 55905
(800) 422-6296
University of Minnesota Medical Center
909 Fulton St. SE
Minneapolis, MN 55455
(612) 273-8383
St. Louis University Hospital
1201 S Grand Blvd
St. Louis, MO 63104
(314) 257-8000
University of Mississippi Medical Center
2500 N State St
Jackson, MS 39216
(601) 984-1000
Atrium Health Transplant Center
1025 Morehead Medical Drive Suite 600
Charlotte, NC 28203
(800) 562-5752
Duke Heart Transplant Clinic, Duke University Medical Center
40 Duke Medicine Cir Clinic 2F/2G
Durham, NC 27710
(919) 681-1300
UNC Hospitals
101 Manning Dr
Chapel Hill, NC 27514
(984) 974-1000
UNC School of Medicine
321 S Columbia St
Chapel Hill, NC 27599
(919) 962-8335
WakeMed Raleigh Campus
3000 New Bern Ave
Raleigh, NC 27610
(919) 350-8000
The Nebraska Medical Center
42nd and Emile
Omaha, NE 68105
(402) 559-4000
Newark Beth Israel Medical Center
201 Lyons Ave
Newark, NJ 07112
(800) 843-2384
Brookdale University Hospital Medical Center
1 Brookdale Plaza
Brooklyn, NY 11212
(718) 240-5000
New York Columbia Presbyterian CUIMC/Milstein Hospital Building
177 Fort Washington Ave
New York, NY 10032
(212) 305-2500
North Shore University Hospital
300 Community Dr
Manhasset, NY 11030
(516) 562-0100
NewYork-Presbyterian / Weill Cornell Medical Center
525 E 68th St
New York, NY 10065
(212) 746-5454
New York – Presbyterian Hospital/Columbia
177 Fort Washington Ave
New York, NY 10032
(800) 227-2762
Langone Hospital Kimmel Pavilion
424 E 34th St
New York, NY 10016
(212) 263-7300
Westchester Medical Center
257 Lafayette Ave # 300
Valhalla, NY 10901
(845) 368-0330
The Lauder Family Cardiovascular Center of Mount Sinai Heart
1190 5th Ave
New York, NY 10029
(855) 674-3278
Donor Memorial Lifeline of Ohio
770 Kinnear Rd
Columbus, OH 43212
(614) 291-5667
Cleveland Clinic Transplant Center
9500 Euclid Ave
Cleveland, OH 44195
(216) 444-6996
Cincinnati Children's Hospital Medical Center
3333 Burnet Ave
Cincinnati, OH 45229
(513) 636-4200
The Christ Hospital
2139 Auburn Ave
Cincinnati, OH 45219
(513) 585-2000
University of Cincinnati Medical Center
3188 Bellevue Avenue
Cincinnati, OH 45219
(513) 584-1000
UH Cleveland Medical Center
11100 Euclid Ave
Cleveland, OH 44106
(216) 541-1758
INTEGRIS Health Baptist Medical Center
3300 Northwest Expy
Oklahoma City, OK 73112
(405) 949-3011
Providence St. Vincent Medical Center
9205 SW Barnes Rd
Portland, OR 97225
(503) 216-1234
Oregon Health & Science University
3181 SW Sam Jackson Park Rd
Portland, OR 97239
(503) 494-8311
Hospital of the University of Pennsylvania - Pavilion
1 Convention Ave
Philadelphia, PA 19104
(215) 662-4000
University of Pittsburgh Medical Center
3459 Fifth Ave
Pittsburgh, PA 15213
(877) 640-6746
Thomas Jefferson University Hospital - Jefferson Health
111 S 11th St
Philadelphia, PA 19107
(215) 955-6000
Pittsburgh VA Medical Center
4100 Allequippa St
Pittsburgh, PA 15240
(412) 822-2222
MUSC Health University Medical Center
171 Ashley Ave
Charleston, SC 29425
(843) 792-1414
Vanderbilt University Medical Center
1215 21st Ave S
Nashville, TN 37232
(615) 936-3500
Baylor Scott & White Medical Center - Plano
4700 Alliance Blvd
Plano, TX 75093
(469) 814-2000
Baylor Scott & White Medical Center — Temple
2401 S 31st St
Temple, TX 76508
(254) 724-2111
Baylor University Medical Center
3500 Gaston Ave
Dallas, TX 75246
(214) 820-0111
St. Luke's Health - Baylor St. Luke's Medical Center
1101 Bates Ave
Houston, TX 77030
(832) 355-1000
Memorial Hermann-Texas Medical Center
6411 Fannin St
Houston, TX 77030
(713) 704-4000
Methodist Heart and Lung Institute Lung Center
7726 Louis Pasteur Dr
San Antonio, TX 78229
(210) 575-9500
Methodist Hospital Stone Oak
1139 E Sonterra Blvd
San Antonio, TX 78258
(210) 638-2000
Baylor Scott & White All Saints Medical Center — Fort Worth
1400 8th Ave
Fort Worth, TX 76104
(817) 926-2544
University Hospital
4502 Medical Drive
MS 18-1
San Antonio, TX 78229
(210) 567-5777
Children's Medical Center Dallas
1935 Medical District D
Dallas, TX 75235
(214) 456-7000
Dell Children's Medical Center
4900 Mueller Blvd
Austin, TX 78723
(512) 324-0000
Medical City Dallas Hospital
7777 Forest Ln
Dallas, TX 75230
(972) 566-7000
Methodist Hospital Specialty and Transplant
8026 Floyd Curl Dr
San Antonio, TX 78229
(210) 575-8110
UT Southwestern Medical Center
5323 Harry Hines Blvd
Dallas, TX 75390
(214) 648-3111
Baylor University Medical Center - Roberts Hospital
Scott & White Health 3500 Gaston Ave
Dallas, TX 75246
(214) 820-0111
William P. Clements Jr. University Hospital
6201 Harry Hines Blvd
Dallas, TX 75235
(214) 633-5555
Intermountain Medical Center
5121 Cottonwood St.
Murray, UT 84107
(801) 507-7000
University of Utah Hospital
50 Medical Dr N
Salt Lake City, UT 84132
(801) 581-2121
DonorConnect
6065 S Fashion Blvd #125
Murray, UT 84107
(801) 521-1755
Inova Fairfax Medical Campus
3300 Gallows Rd
Falls Church, VA 22042
(703) 776-4001
VCU Medical Center
1200 E Marshall St
Richmond, VA 23219
(804) 828-9000
VCU Health Hume-Lee Transplant Center
1200 E Marshall St 7th Floor
Richmond, VA 23219
(804) 828-4104
LifeCenter Northwest
3650 131st Ave SE
Bellevue, WA 98006
(877) 275-5269
UW Medical Center - Montlake
9725 3rd Ave NE #400
Seattle, WA 98195
(206) 598-3300
UW Medical Center - Northwest
1550 N 115th St Main Hospital
Seattle, WA 98133
(877) 694-4677
University of Washington School of Medicine
1959 NE Pacific St
Seattle, WA 98195
(206) 543-2100
Froedtert Luth Memorial Hospital
9200 W. Wisconsin Ave
Milwaukee, WI 53226
(414) 777-7700
Aurora St Luke's Medical Center
2900 W Oklahoma Ave
Milwaukee, WI 53215
(414) 649-6000
1. Expanded criteria lungs is defined as donor lung pairs initially deemed unacceptable for procurement and transplantation based on limitations of cold static preservation.
2. Expanded criteria hearts is defined as donor hearts that are deemed unsuitable for procurement and transplantation at initial evaluation due to limitations of prolonged cold static cardioplegic preservation (e.g., > 4 hours of cross-clamp time).
3. DCD livers < 55 years old with < 30 mins warm ischemia time and macrosteatosis < 15%.

Only multi-organ platform used with donor lungs, hearts and livers

Largest user base at more than 70 leading US and global transplant centers

Largest body of literature and clinical evidence in warm perfusion

Most extensive training program including 24/7 clinical support